全港首家
定位導航腦磁激中心
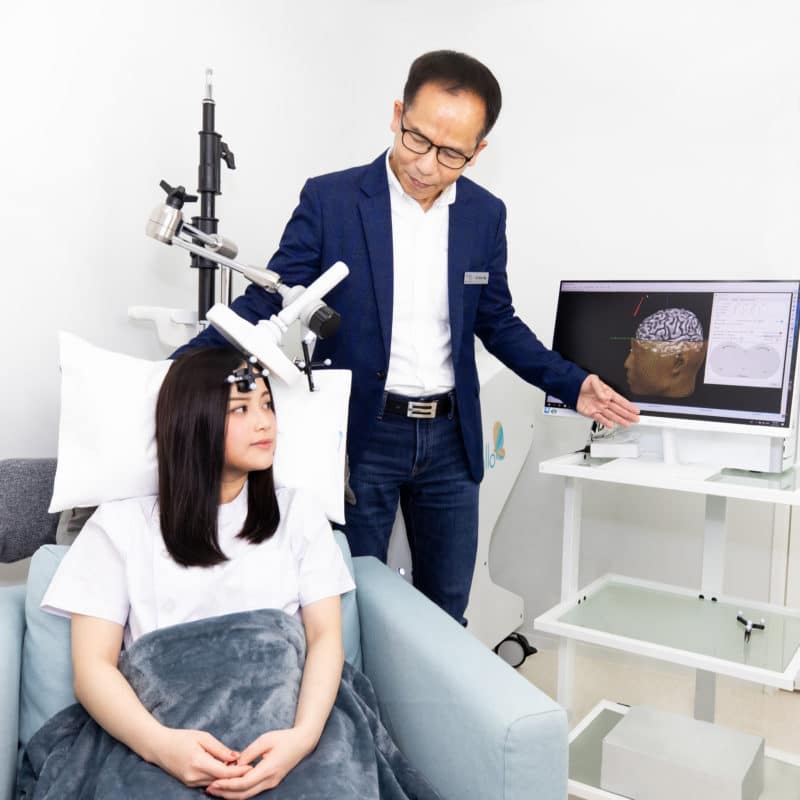
作為全港首家配備定位導航技術的腦磁激中心,本中心設有德國先進 MAG & More Apollo nTMS 設備,配合NDI Polaris Vicra 遙距立體大腦定位導航(腦外科手術常用系統),應用於治療腦部相關的疾病,成效得到權威機構美國FDA及歐盟的CE認證,優雅簡潔的設計亦令療程變得更舒適更方便!
我們會保證您有滿意的治療體驗!
治療範圍
全球不少頂尖的大學和醫院已進行不同類型的醫學研究,臨床結果發現具導航功能的腦磁激療法能有效治療多種腦部相關的疾病,當中包括:
- 抑鬱症
- 焦慮病症
- 腦退化症/阿茲海默症
- 失眠
- 耳嗚
- 慢性神經痛
- 纖維肌痛
- 柏金遜症
- 幻聽
- 腦中風康復治療
*部分為off-label治療方法:醫療研究證明有效,正在等待更多研究報告及官方審核。
甚麽是定位導航腦磁激?
定位導航腦磁激(nTMS) 是一項安全、有效、非入侵性的醫療新科技,近年獲歐美認可廣泛應用於治療腦部相關的疾病,成效顯著:
- TMS利用磁電交換轉化產生磁性脈衝的原理,針對大腦與疾病關聯的區域進行刺激,有效改善患者的腦部功能和情緒
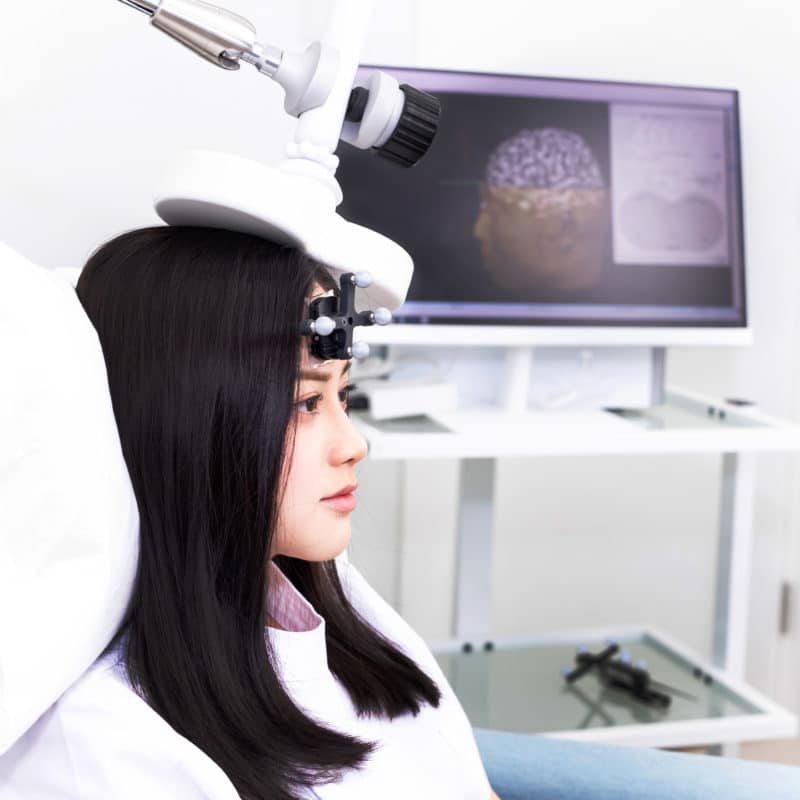
- TMS的治療部位通常極為微小,定位導航腦磁激(Navigated TMS)可以把握TMS治療的關鍵之處,準確地刺激相關的神經組織
- 沒有導航技術的TMS治療系統只能單靠過往科研文獻粗略估計出刺激部位,可能影響治療效果
本中心的腦磁激系統配合磁力共振(MRI)的3D大腦影像進行導航,將磁性脈衝能量精確集中地瞄準大腦的正確位置,為客人提供個人化、有效和安全性極高的腦磁激治療。
nTMS治療方案
現時,世界各地的知名大學正在進行大型的臨床研究去改進腦磁激技術。其中一個被廣泛認可的用途是用來醫治抑鬱症的治療方案:
- 整個療程持續4-8星期
- 每一星期三至五次
- 每次療程持續大約19-37分鐘
- 過程病人不用麻醉,完全保持清醒

腦磁激設備由受訓醫療人員操作。本治療中心同時提供相關的專業人士的意見和協助,如需查詢,請與本中心聯絡。
* FDA 認證是指美國食品藥品監督管理局已對相關醫療設備的臨床效益和風險進行了仔細評估,並且確認該技術已得到大量臨床數據支持。
**CE 認證是由歐盟發出的安全認證,證明產品不會危及人類安全和健康。
nTMS 治療流程

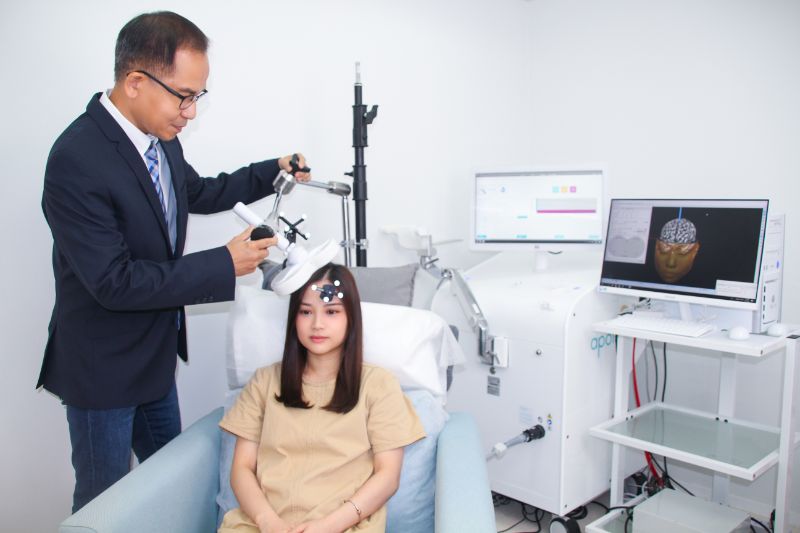
立體腦成像 (3D Brain Imaging)
系統會透過患者的MRI掃描建立一個專屬客人的3D頭部模型。

最佳治療強度 (Optimal Treatment Intensity)
在建立客人的立體大腦成像模型後,治療人員會通過肌電圖(EMG)中顯示的數值為客人計算最佳的治療強度。

定位導航 (Brain Navigation)
透過立體大腦成像模型中,利用定位導航技術尋找並確定特定需要刺激的區域。

穩定有效的治療體驗 (Consistent and Effective Treatment Experience)
在設定正確的刺激位置後,便可開始TMS治療。在完成第一次治療後,系統會自動儲存患者的需要刺激的位置及強度,令患者之後都可以接受最穩定、無偏差的TMS治療。
BrainVision nTMS
為您提供獨一無二的治療體驗
本治療中心設有 MAG & More APOLLO nTMS系統能夠真正地為患者提供個人化以及精準的腦磁激治療。
在這裡,您可以找到與坊間腦磁激中心不同的治療體驗!

定位導航nTMS治療的特點:

非入侵性
不涉及手術、麻醉或鎮靜藥物,患者在治療期間保持清醒和知覺

定位導航技術
利用立體磁力共振 (3D-MRI) 的立體大腦影像進行定位,達至準確和個人化的治療效果。

國際安全及成效認證
TMS技術已得到美國食品藥品監督管理局 (U.S.Food and Drug Administration, FDA) 許可及歐盟發出的歐洲合格認證(CE-marked),安全性及其治療成效得到確認。

安全無創
由於TMS只是以磁力改變大腦的神經活動,不會改變患者身體其他化學成份,因此不會出現藥物的副作用,例如體重增加,疲勞等等。
最舒適,最有效的治療體驗
TMS 以無創的磁性脈衝改善神經活動,專家科研實證能有效提昇情緒,改善記憶和認知能力,以及治療其他腦部相關疾病,例如抑鬱症、焦慮症、腦退化症、耳鳴和帕金遜症等等,成效非常顯著。
相比其他同類型的TMS儀器,研究指出Apollo TMS 發出極低聲量,讓你可以放鬆心情在寧靜舒適的環境進行療程。療程一般持續19至37分鐘,完成治療後,可立刻回復正常工作及活動。
配備獨家定位導航技術
我們系統的另一優勝之處是它的定位導航技術,透過客人的磁力共振腦掃瞄,構建立體大腦模型,令腦磁激成為一種「看得見」的治療,儀器可以精確地瞄準大腦正確位置,進行針對性的刺激,治療效果特別優勝。
「度身訂造」的療程
專業醫學團隊主理
本中心團隊包括臨床心理學家、腦神經心理學家及精神專科醫生提供診斷和咨詢,療程由在德國受訓的專業醫療人員主理,信心保證。

定位導航 Apollo TMS ™ 與其他腦磁激系統比較
| 特點比較 | 沒有導航技術的腦磁激系統 | 定位導航 Apollo 腦磁激 |
|---|---|---|
| 磁力共振(MRI)大腦掃描定位 | ||
| 專屬個人化腦傳導模型 | ||
| 準確量度肌肉反應的肌電圖(EMG) | ||
| 國際成效及安全認證(FDA/CE) | ||
| 具靈活性的頭頸支撐(Head-Neck Support) | ||
| 單一磁性脈衝/重覆性腦磁激功能 | ||
| 系統操作寧靜度 | 研究發現APOLLO腦磁激系統操作時最為寧靜 |
您有抑鬱焦慮問題嗎?
讓我們了解您的情況,為你尋找最適切的治療!
常見問題
與普通的腦磁激系統不同,Apollo Navigated TMS系統是配備精準的導航科技,配合數碼立體的腦成像功能,得出患者大腦的磁力其振成像(MRI),並將其影像上傳到系統之中,令導航系統可以避免單靠猜測來設定需要刺激的大腦區域,而是針對性地瞄準特定的區域進行刺激,為患者提供最精準、最個人化、最有效和安全性極高的腦磁激治療。
相反,普通的腦磁激系統沒有配備導航系統,只能使用頭皮的特定測量值估量需要磁激的位置,要針對大腦其他部位就更加困難。所以沒有配備導航的腦磁激系統會有更高的誤差,可能未能達到理想的治療效果。
在治療抑鬱症上,腦磁激技術早在2008年已經通過美國食品藥物管理局(FDA)的安全認可。大量大規模的臨床研究而證明腦磁激是一種無創並且有效的抑鬱症治療,安全性極高。
現時,用以腦磁激技術治療腦退化症(Alzheimer’s Disease) 已申請美國食品藥物管理局的安全認可(Pending FDA-approval),相信會在年底有結果。
用腦磁激技術來醫治其他精神科或神經系統疾病,例如焦慮病症、神經性疼痛、腦中風、耳鳴及腦中風康復治,大部份都有臨床研究證據支持,技術亦得到由歐盟發出的歐洲合格認證(CE-marked),安全性得到保證。
一般治療時間連同所有預備工作大約在25-40分鐘內完成。患者會舒適地坐在椅子上,治療中心會播放音樂和影片令患者放鬆,患者全程保持完全清醒和知覺。
在腦磁激治療過程中,患者不會感受到很大的痛楚。患者會感受到儀器在頭皮上有輕微的輕敲感,少數患者會感到輕度的頭皮壓痛,但這些感覺在療程期後就會很快消失。在程序結束後,患者可以立即恢復正常活動。
在過往的臨床醫學研究中,一般患者每週接受5次的腦磁激治療,持續 19 - 37 分鐘,持續 4 - 6 週,一般患者在治療第4週症狀就會開始得到緩解。在療程完成後,要根據精神科醫生及臨床心理學家的判斷去決定患者是否需要進行後續治療。
在過往的臨床研究中,腦磁激療法可以與抗抑鬱藥一起安全使用,並無不良後果。至於與其他精神科藥物的一併使用,暫時未有研究結果證實,但使用前請與你的精神科醫生商討。
